From groundbreaking research and development to successful product launches, our stories showcase our unwavering commitment to improving global healthcare. Our multidisciplinary approach and state-of-the-art facilities drive us forward, delivering impactful solutions to patients in need and defining our path to excellence.

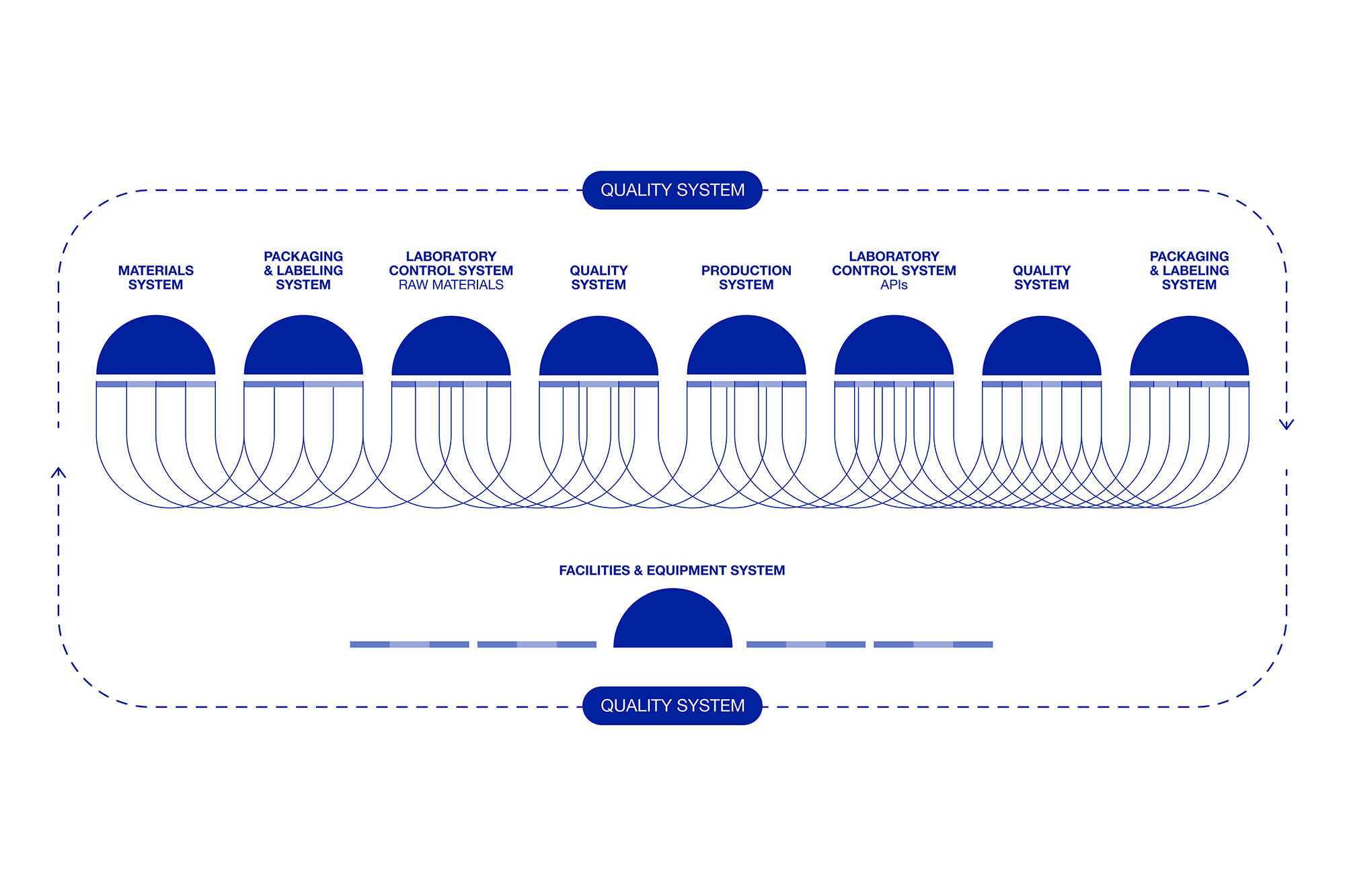
The Pharmaceutical Quality System (PQS) is a management framework ensuring consistent production of high-quality products.

At Seratec, quality is not just a goal—it’s the foundation of everything we do and embedded throughout the entire product life cycle.
The FDA ensures all pharmaceutical products, including APIs, meet top quality and safety standards.
An active pharmaceutical ingredient (API) is a substance or mixture used in medicine production, acting as the component responsible for therapeutic effects.

Quality at Seratec plays a crucial, cross-functional role. For us, quality isn’t just a goal—it’s a driving force behind everything we do.

Seratec specializes in areas ranging from developing active molecules to offering turnkey solutions for APIs.
After many years of formation, Seratec aims to implement 21st century GMP tools in which the keystone is combining an understanding of the product and the procedure, and their variability, in a new pharmaceutical paradigm. These ambitions are reflected in our approach to excellence in business, in which new concepts of development and manufacturing of active pharmaceutical ingredients, process validation, statistical control of processes, and the integration of the pharmaceutical quality system are organized… just to name a few.
