An active pharmaceutical ingredient, or API, is any substance or mixture intended for use in the production of a medicinal product, becoming the active component responsible for its therapeutic effects. APIs are designed to have pharmacological activity or other direct effects on diagnosing, curing, treating, or preventing diseases, as well as influencing the structure or function of the human body.
Medicinal products typically consist of one or more active ingredients—often in small amounts—and various non-active substances, or excipients, which aid in shaping the medicine (e.g., tablets, capsules) and influencing how the API interacts with the body.
At Seratec, we specialize in producing APIs through organic synthesis, a process that transforms raw materials into active pharmaceutical compounds. Unlike APIs derived from natural sources or biotechnology, Seratec’s APIs are created through precise chemical reactions. These reactions typically occur in reactors, where raw materials and reagents are combined in a liquid medium (solvents). The resulting products—whether intermediate compounds or final APIs—are then isolated, often as powders, and distributed for further processing by pharmaceutical companies into finished medicinal products.
The pharmaceutical industry is one of the most highly regulated sectors worldwide. Strict international regulations ensure the quality, safety, and effectiveness of medicinal products. Good Manufacturing Practices (GMP), or current Good Manufacturing Practices (cGMP), are quality standards designed to ensure that medicines are consistently produced and controlled according to strict guidelines.
A medicinal product can only be marketed after obtaining a Marketing Authorization (MA) from health authorities, confirming the product’s safety and efficacy. The Marketing Authorization Holder (MaH) is responsible for ensuring that the medicine complies with these regulatory requirements. Since the API is the active component, its design, manufacture, and analysis must also meet GMP standards. This information is documented in an Active Substance Master File (ASMF) in Europe or a Drug Master File (DMF) in the U.S., which provides detailed information on the API’s chemistry, manufacturing process, and quality control measures.
Seratec plays a critical role in the pharmaceutical industry by creating and registering API files with regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and regulatory bodies across Asia. We ensure that our APIs are produced in full compliance with GMP, especially in niche markets where we hold the master file.
Beyond adhering to global regulatory standards, Seratec is committed to driving innovation in API development. We continually invest in research and development to enhance our manufacturing processes, ensuring the production of APIs that meet evolving industry demands. By adopting cutting-edge technologies and refining chemical synthesis techniques, Seratec not only maintains high product quality but also improves efficiency and sustainability in API production. This ongoing commitment positions Seratec as a leader in the pharmaceutical industry, delivering safe and effective APIs that serve patients around the world.

The Pharmaceutical Quality System (PQS) is a management framework ensuring consistent production of high-quality products.
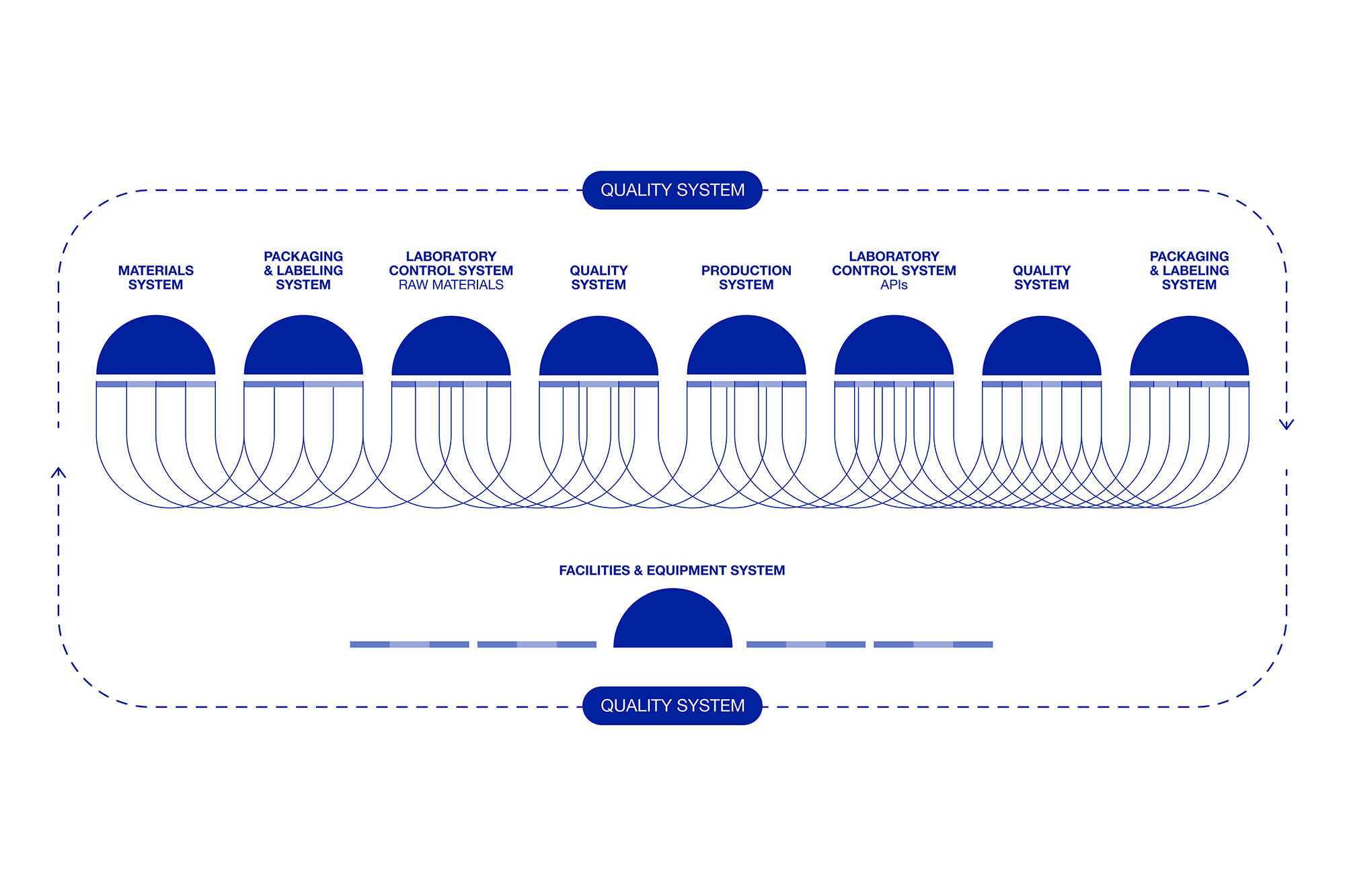
The FDA ensures all pharmaceutical products, including APIs, meet top quality and safety standards.

Seratec's strength is our deep understanding of operations and the pharmaceutical industry, setting us apart in a competitive market.